Page 26 - ChemLife Sayı 23
P. 26
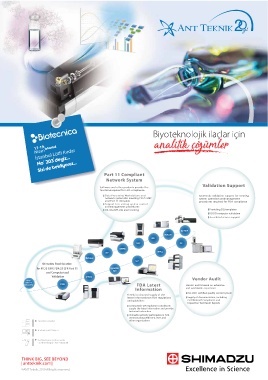
Biyoteknolojik ilaçlar için
17-19 İstanbul analitik çözümler
İstanbul Lütfi Kırdar
Nisan
No° 203’deyiz...
Sizi de bekliyoruz...
Part 11 Compliant
Network System
Validation Support
Software and other products provide the
functionsrequired for FDA compliance.
Data Processing Workstations and Systematic validation support for creating
network systemsfor meeting PIC/S GMP system operation andmanagement
and Part 11 demands procedures required for FDA compliance
Support for creating system control
and management procedures
On-site/o -site user training Providing DQ templates
IQ/OQ computer validation
Accredited service support
Q-TOF
MALDI
MS
TOC GC
ICPMS
ICP HPLC
Balance
UV
Shimadzu Total Solution
for PIC/S GMP, FDA 21 CFR Part 11 Aggregates Vendor Audit
Shimadzu Total Solution Sizer
and Computerized Vendor audits based on extensive
for PIC/S GMP, FDA 21 CFR Part 11 PPSQ and worldwide experience
Validation
ISO-9001 certi ed quality control system
Other and Computerized Vendor Audit
vendor’s Supply of documentation, including Certi cates
instruments FTIR FDA Latest Vendor audits based on extensive-
Validation
Information and worldwide experience
on FDA regulations and guidelines
ISO-9001 certi ed quality control system
Timely issuing and supply of the
latest informationon FDA regulations Supply of documentation, including
and guidelines Certi catesof Compliance and
Inspection Test Result Reports
Contracted FDA regulation consultants
supply the latest information and provide
technical instruction
Shimadzu actively participates in FDA
seminarsalong with ISPE, PDA, and
other organizations
©ANT Teknik, 2019 All rights reserved.